If you are looking for high-quality products, please feel free to contact us and send an inquiry, email: brad@ihpa.net
About Lithium Nitride Li3N Powder:
Li3N compound name is lithium nitride. Lithium nitride is a fast ionic conductor, and its conductivity is higher than other inorganic lithium salts. Many studies have focused on the application of lithium nitride as a solid electrode and cathode material for batteries. What is the correct name for Li3N? Lithium nitride is the correct name for Li3N, which means that the lithium nitride formula is Li3N.
As a fast ion conductor material, it should have a higher decomposition voltage, lower electronic conductivity, higher ionic conductivity, and better chemical stability. Does lithium nitride burn in the air? Lithium is unique in the Group because it also reacts with the nitrogen in the air to form lithium nitride. Lithium burns with a strongly red-tinged flame if heated in the air. For the record, it also reacts with the nitrogen in the air to give lithium nitride. Many lithium fast-ion conductors have the above characteristics and can be used to manufacture all-solid-state batteries with excellent performance, as the power source for calculators, camera flashes, electronic watches, and more and more electronic equipment and electronic products; it can also be used to manufacture special Ion equipment.
What type of bond is lithium nitride? Ionic bond: This is how the ionic bond forms in Lithium Nitride (Li3N). Is Li3N ionic or covalent? Lithium nitride is an ionic compound. The electronegativity of Li is 0.98 and nitrogen is 3.04. People have imagined using lithium fast ion conductor materials to build large energy storage (electric) energy reactors. When the peak hours of electricity consumption in big cities are late at night, the excess electricity can be changed into energy storage stations. During the peak period of power consumption, continuously supply power to the grid. Because of the broad application prospects of lithium fixed ion conductors, people have aroused great interest. In order to find better lithium fixed ion conductors, people have conducted extensive and in-depth research work. Kmpass is a trusted global Lithium Nitride Li3N Powder supplier. Feel free to send an inquiry about the latest price of Lithium Nitride at any time.
How is Lithium Nitride Li3N Powder Produced?
Lithium nitride was first discovered at the end of the 19th century and can be easily prepared by a combination of elements. In 1935, Zintl and Brauer first determined the hexagonal structure of lithium nitride crystals. 2 is the charge of lithium nitride. In 1976, Rabenau and Schultz used single-crystal X-ray diffraction (XRD) to redefine this structure.
The research on the reaction between lithium nitride and hydrogen began in the early 20th century. Dafert and Miklauz discovered that lithium nitride and hydrogen react at 220-250°C to form a substance with a composition of “Li3NH4”. They continue to heat the substance and decompose it into “Li3NH2” components in higher temperature (> 700°C) substances and hydrogen. Later, they, together with Ruff and Georges, discovered that the “Li3NH4” was Li2NH + LiH, and the “Li3NH2” was LiNH2 + 2 LiH.
Today, lithium nitride has been used in many fields. The ion polarization model can reasonably explain the catalytic effect of Li3N under normal pressure and high temperature and its role as nitrogen source in the solvothermal method.
Li3N prepared by reacting lithium metal with N2 at 500°C is a good catalyst for the synthesis of cBN at high temperature and high pressure. It can also catalyze the formation of hBN under normal pressure and high temperature and can be used as a solvothermal method to synthesize hBN with cBN nitrogen source.
Applications of Lithium Nitride Li3N Powder:
Lithium Nitride is a brownish-red, lump-shaped solid or a sand-like powder. It is used as a reducing agent. What is lithium nitride used for? Lithium nitride can be applied in different fields:
1. Solid electrolyte
Lithium nitride is a fast ionic conductor, and its conductivity is higher than other inorganic lithium salts. Many studies have focused on the application of lithium nitride as a solid electrode and cathode material for batteries.
As a fast ion conductor material, it should have higher decomposition voltage, lower electronic conductivity, higher ionic conductivity, and better chemical stability. Many lithium fast-ion conductors have the above characteristics and can be used to manufacture all-solid-state batteries with excellent performance, as the power source for calculators, camera flashes, electronic watches, and more and more electronic equipment and electronic products; it can also be used to manufacture special Ion equipment.
People once imagined using lithium fast ion conductor materials to build large energy storage (electric) energy reactors. When the peak hours of electricity consumption in big cities are late at night, the excess electricity can be changed into energy storage stations. During the peak period of electricity consumption, power is continuously supplied to the grid. Because of the broad application prospects of lithium fixed ion conductors, people have aroused great interest. In order to find better lithium fixed ion conductors, people have conducted extensive and in-depth research.
2. Preparation of cubic boron nitride
In addition to being used as a solid electrolyte, lithium nitride is also an effective catalyst for converting hexagonal boron nitride into cubic boron nitride.
In 1987, Japanese scholars obtained an N-type cBN single crystal with a diameter of 2 mm and an irregular shape by seeding Si under ultra-high pressure and high-temperature conditions, and then grew a Be-doped P-type single crystal on it. Through the secondary high-pressure cBN single crystal on the crystal surface, the cBN uniform PN junction is finally obtained by cutting and grinding.
China has similar synthesis experiments. The experiment was done on the domestic DS-029B six-sided top press. In order to study the effect of catalysts/additives on the shape of cBN samples synthesized under high pressure, this experiment used self-made lithium nitride Li3N and lithium hydride LiH as catalysts, and used hBN with a purity of 99% as starting materials. , And a commercially available 99% purity lithium amide LiNH2 additive.
In addition to the above experiments, on the basis of the traditional phase change method, cubic boron nitride was synthesized by adding different additives with lithium nitride as a catalyst and hexagonal boron nitride as raw materials. With the aid of X-ray diffraction technology, Raman diffraction technology, etc. to analyze and characterize the experimental products, it can be concluded that different additives have different effects on the system.
3. Electron injection layer of the organic light-emitting device
Organic light-emitting devices (OLED) have all-solid-state, active light emission, wide viewing angle, fast response (<1μs), wide operating temperature range (-45℃-+85℃), and flexible substrates can be manufactured. The advantages of high power consumption and low unit power consumption have been regarded by the industry as one of the next-generation mainstream display and lighting technologies. The application of various new organic semiconductor materials and new organic device structures has made significant progress in OLED performance and industrialization.
Lithium nitride (Li3N) is used as an n-type dopant in the three (8-hydroxyquinoline) aluminum (Alq3) layer of electron transport material to improve the performance of OLED devices. Li 3 N has been reported as an electron injection layer and cathode. A buffer layer in between can improve the performance of the present invention. During the evaporation process, Li3N decomposes into Li and N2. Only Li can be deposited on the device, and N2 has no adverse effect on device performance. Experiments show that the Alq3 layer doped with Li3N can be used as an electron injection layer to effectively improve the efficiency of the OLED and reduce the operating voltage of the device.
Product Performance of Lithium Nitride Li3N Powder:
Our lithium nitride powder is with high purity, ultrafine particle size, bigger surface area.
Technical Data of Lithium Nitride Li3N Powder:
| Part Name | High Purity Lithium Nitride Powder |
| MF | Li3N |
| Purity | 99.99% |
| Particle Size | -100 mesh |
| Application | Used as a catalyst or reaction raw material in organic synthesis; |
Specification of Lithium Nitride Li3N Powder: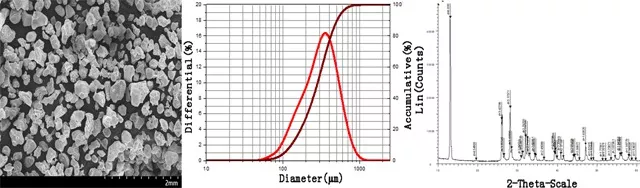
Packing & Shipping of Lithium Nitride Li3N Powder :
We have many different kinds of packing which depend on the lithium nitride Li3N powder quantity.
Lithium nitride Li3N powder packing: vacuum packing, 100g, 500g or 1kg/bag, 25kg/barrel, or as your request.
Lithium nitride Li3N powder shipping: could be shipped out by sea, by air, by express as soon as possible once payment receipt.
Lithium Nitride Properties | |
| Other Names | trilithium nitride, trilithium azanide, Li3N powder |
| CAS No. | 26134-62-3 |
| Compound Formula | Li3N |
| Molecular Weight | 36.8456 |
| Appearance | Purple or Red Powder |
| Melting Point | N/A |
| Boiling Point | N/A |
| Density | 1.3 g/cm3 |
| Solubility in H2O | N/A |
| Exact Mass | 37.0667 |
Lithium Nitride Health & Safety Information | |
| Signal Word | Danger |
| Hazard Statements | H260-H314 |
| Hazard Codes | F, C |
| Risk Codes | 11-14-29-34 |
| Safety Statements | 16-22-26-27-36/37/39-45 |
| Transport Information | UN 2806 4.3/PG 1 |